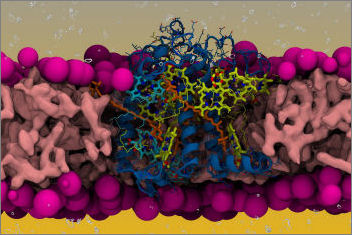
Light-harvesting complexes can transfer excitation energy to the reaction centres when in a light-harvesting regime, and instead dissipate the absorbed light as heat when in a quenching regime induced under high-light conditions. Conformational rearrangements of the protein matrix are thought to play a key role in affecting the interactions of the bound pigments, allowing or disallowing energy transfer between them and therefore changing the activity regime. However, the identity of such conformations is not known. In our Nature Communications paper we integrate an enhanced sampling approach based on replica exchange molecular dynamics (REMD) and metadynamics (MetaD) with quantum mechanical (QM) calculations to shed light on the connection between protein dynamics and pigment-pigment interactions in the minor light-harvesting complex of higher plants CP29. We are able to identify the main conformational rearrangements on the lumenal side of the complex, and to rule out the possibility of tuning the onset of NPQ via the Coulomb coupling between the most coupled carotenoid-chlorophyll pairs. We provide insights that short-range interactions instead can more easily be tuned by the protein dynamics than long-range Coulomb interactions.