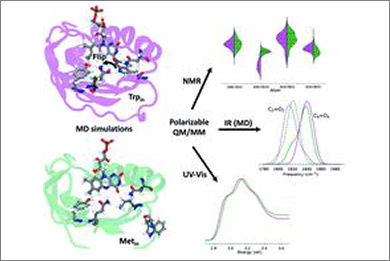
Photoreceptor proteins bind a chromophore, which, upon light absorption, modifies its geometry or its interactions with the protein, finally inducing the structural change needed to switch the protein from an inactive to an active or signaling state. In the Blue Light-Using Flavin (BLUF) family of photoreceptors, the chromophore is a flavin and the changes have been connected with a rearrangement of the hydrogen bond network around it on the basis of spectroscopic changes measured for the dark-to-light conversion. However, the exact conformational change triggered by the photoexcitation is still elusive mainly because a clear consensus on the identity not only of the light activated state but also of the dark one has not been achieved. Here, we present an integrated investigation that combines microsecond MD simulations starting from the two conflicting crystal structures available for the AppA BLUF domain with calculations of NMR, IR and UV-Vis spectra using a polarizable QM/MM approach. Thanks to such a combined analysis of the three different spectroscopic responses, a robust characterization of the structure of the dark state in solution is given together with the uncovering of important flaws of the most popular molecular mechanisms present in the literature for the dark-to-light activation.
Hashem, S.; Macaluso, V.; Nottoli, M.; Lipparini, F.; Cupellini, L. & Mennucci, B.
Chem. Sci. 12, 13331-13342 (2021) https://doi.org/10.1039/d1sc03000k