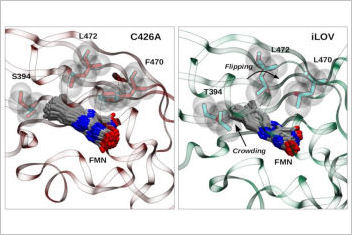
Studies in cell biology often depend on imaging techniques with high spatial and temporal resolution. To this end, many artificial fluorescent protein were developed and are widely employed as molecular reporters. We analyzed two synthetic flavin-based fluorescent proteins, derived from natural light, oxygen, and voltage (LOV) sensing domain, which were engineered to obtain improved fluorescence properties. We compared a single mutant protein C426A used for us as a reference system with the popular biomarker iLOV. The iLOV protein has optimized protein-chromophore van der Waals interactions with respect to the single mutant protein (C426A). In contrast, the h-bonding dynamics around the flavin has little influence on the fluorescence-efficiency tuning. On the contrary, increase in fluorescence from C426A to iLOV is probably due to the better packing of the flavin in the iLOV biding-pocket that involves a “crowding and flipping” mechanism, which was proposed by crystallographic studies but it had not yet been studied systematically through simulations.
Ramos, F. C.; Cupellini, L. & Mennucci, B.
J. Phys. Chem. B 125,1768-1777 (2021) https://pubs.acs.org/doi/10.1021/acs.jpcb.0c10834