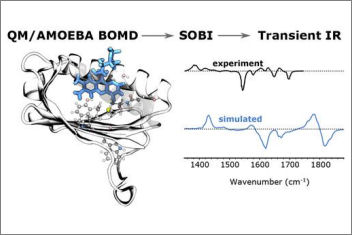
Ultrafast transient infrared spectroscopy is a powerful technique that not only indicates the dynamic structural changes of protein-bound chromophores involved in a photochemical reaction but also reveals the implications of those parts of the protein that contribute in that reaction. In our J. Phys. Chem. paper, we design a novel strategy to compute the transient IR spectra of a protein-bound chromophore using multiscale polarizable QM/MM MDs of both ground and excited states trajectories of the protein. We apply this technique to AppA, a blue-light-utilizing flavin protein domain. Additionally, we employ a signaling processing technique (SOBI) to assign the IR peaks, which allowed a clear assignment of the transient signals to the normal modes of the flavin ring.