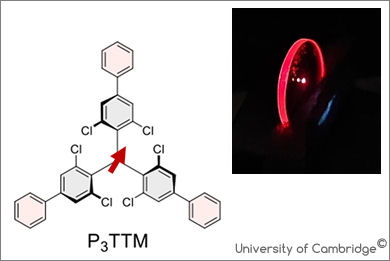
Organic radicals based on tris(2,4,6-trichlorophenyl)methyl (TTM) radicals show efficient photoluminescence from excitons in the spin-doublet manifold, but their potential in charge photogeneration remains unexplored. Here we report that when TTMs are in contact, photoexcitation generates TTM anion–TTM cation pairs. These can decay radiatively or be fully separated under an electric field bias. We use a triphenyl-substituted TTM (P3TTM) in which the phenyl end groups enhance intermolecular interactions. In dilute (5 wt%) films in a wide-energy-gap organic semiconductor host, we observe prompt photoluminescence from the excited radical at 645 nm, and a delayed component, beyond 1 μs, at 800 nm due to recombination of P3TTM anion–cation pairs. Measurements of photocurrent made with diode structures with 100% P3TTM showed close-to-unity charge collection efficiency in reverse bias. We have found ‘homojunction’ intermolecular charge separation, made possible when the extra energy for double occupancy of the non-bonding radical level on the anion is lower than the energy of the doublet exciton. This opens possibilities for light harvesting using single-material molecular semiconductors.
Li, B.; Murto, P.; Chowdhury, R.; Brown, L.; Han, Y.; Londi, G.; Beljonne, D.; Bronstein, H. & Friend, R. H.
Intrinsic intermolecular photoinduced charge separation in organic radical semiconductors
Nature Materials, 2025
Read the full paper here: https://www.nature.com/articles/s41563-025-02362-z