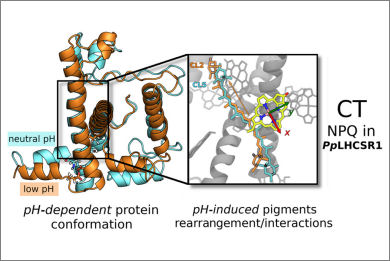
In response to varying light conditions, light-harvesting complexes (LHCs) switch from a light-harvesting state to a quenched state to protect the photosynthetic organism from excessive light irradiation in a strategy known as nonphotochemical quenching (NPQ). NPQ is activated by an acidification of the thylakoid lumen, which is sensed directly or indirectly by the LHC, resulting in a conformational change of the complex that leads to the quenched state. The conformational changes responsible for NPQ activation and their connection to specific quenching mechanisms are still unknown. Here, we investigate the pH-triggered conformational changes in the light-harvesting complex stress-related (LHCSR) of mosses. By combining constant-pH molecular dynamics and enhanced sampling techniques, we find that the pH sensitivity of the complex is driven by the coupled protonation of three residues modulating the conformation of the short amphipathic helix placed at the lumen side of the embedding membrane. Combining these results with quantum mechanics/molecular mechanics calculations, we show that the quenching mechanism sensitive to the pH goes through a charge-transfer between a carotenoid and an excited chlorophyll, which is controlled by the protein conformation.
Pedraza-González, L.; Cignoni, E.; D'Ascenzi, J.; Cupellini, L. & Mennucci, B.
J. Am. Chem. Soc. 145, 13, 7482–7494 (2023) https://pubs.acs.org/doi/full/10.1021/jacs.3c00377