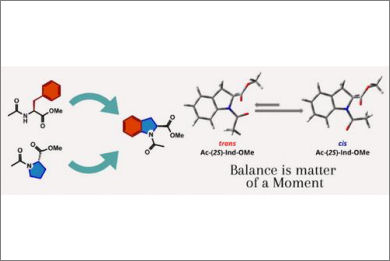
A thorough experimental and computational study on the conformational properties of (S)-indoline-2-carboxylic acid derivatives has been conducted. Methyl (S)-1-acetylindoline-2-carboxylate, both a mimetic of proline and phenylalanine, shows a remarkable tendency toward the cis amide isomer when dissolved in polar solvents. This behavior is opposite to the general preference of proline for the trans isomer, making indoline-2-carboxylic acid a good candidate for the design of different secondary structures and new materials.
Pollastrini, M.; Lipparini, F.; Pasquinelli, L.; Balzano, F.; Barretta, G. U.; Pescitelli, G. & Angelici, G.
J. Org. Chem. 86, 7946-7954 (2021) https://doi.org/10.1021/acs.joc.1c00184