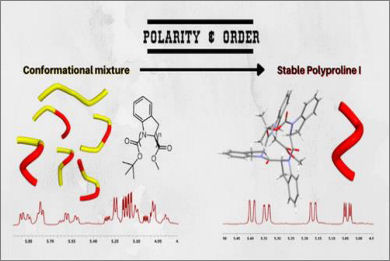
Polyproline I helical structures are often considered as the hidden face of their most famous geminal sibling, Polyproline II, as PPI is generally spotted only within a conformational equilibrium. We designed and synthesized a stable Polyproline I structure exploiting the striking tendency of (S)-indoline-2-carboxylic acid to drive the peptide bond conformation toward the cis amide isomer, when dissolved in polar solvents. The cooperative effect of only four amino acidic units is sufficient to form a preferential structure in solution. We shed light on this rare secondary structure with a thorough analysis of the spectroscopic and chiroptical properties of the tetramer, supported by X-ray crystallography and computational studies.
Pollastrini, M.; Pasquinelli, L.; Górecki, M.; Balzano, F.; Cupellini, L.; Lipparini, F.; Uccello Barretta, G.; Marchetti, F.; Pescitelli, G. & Angelici, G.
J. Org. Chem. 87,13715-13725 (2022) https://doi.org/10.1021/acs.joc.2c01377