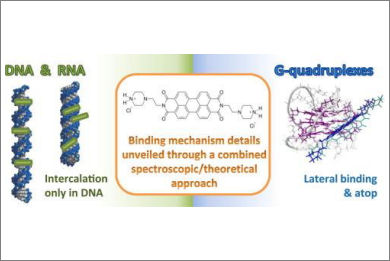
We present here a combined spectroscopic and theoretical analysis of the binding of N,N’-bis(2-(1-piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic acid diimide dichloride (PZPERY) to different biosubstrates. Absorbance titrations and circular dichroism experiments, melting studies and isothermal calorimetry (ITC) titrations reveal a picture where the binding to natural double-stranded DNA is very different from that to double and triple-stranded RNAs (poly(A)∙poly(U) and poly(U)∙poly(A)⁎poly(U)). As confirmed also by the structural and energetic details clarified by density functional theory (DFT) calculations, intercalation occurs for DNA, with a process driven by the combination of aggregates disruption and monomers intercalation. Oppositely, for RNAs, no intercalation but groove binding with the formation of supramolecular aggregates is observed. Among all the tested biosubstrates, the affinity of PZPERY towards DNA G-quadruplexes (G4) is the greatest one with a preference for human telomeric G4s. Focusing on hybrid G4 forms, either sitting-atop (“tetrad-parallel”) or lateral (“groove-parallel”) binding modes were considered in the discussion of the experimental results and molecular dynamics (MD) simulations. Both turned out to be possible concurrently, in agreement also with the experimental binding stoichiometries higher than 2:1.
Macii, F.; Cupellini, L.; Stifano, M.; Santolaya, J.; Pérez-Arnaiz, C.; Pucci, A.; Barone, G.; García, B.; Busto, N. & Biver, T.
Spectrochim. Acta - A: Mol. Biomol. Spectrosc. 260, 119914 (2021) https://doi.org/10.1016/j.saa.2021.119914