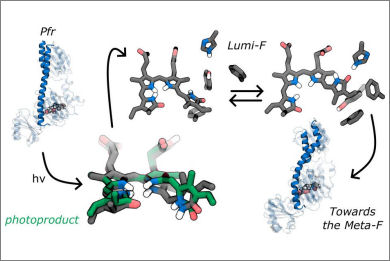
Bacteriophytochromes are light-sensing biological machines that switch between two photoreversible states, Pr and Pfr. Their relative stability is opposite in canonical and bathy bacteriophytochromes, but in both cases the switch between them is triggered by the photoisomerization of an embedded bilin chromophore. We applied an integrated multiscale strategy of excited-state QM/MM nonadiabatic dynamics and (QM/)MM molecular dynamics simulations with enhanced sampling techniques to the Agrobacterium fabrum bathy phytochrome and compared the results with those obtained for the canonical phytochrome Deinococcus radiodurans. Contrary to what recently suggested, we found that photoactivation in both phytochromes is triggered by the same hula-twist motion of the bilin chromophore. However, only in the bathy phytochrome, the bilin reaches the final rotated structure already in the first intermediate. This allows a reorientation of the binding pocket in a microsecond time scale, which can propagate through the entire protein causing the spine to tilt.
Salvadori, G. & Mennucci, B.
The Journal of Physical Chemistry Letters 15 (31), 8078-8084 (2024) https://pubs.acs.org/doi/10.1021/acs.jpclett.4c01823